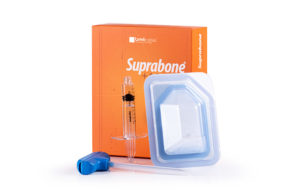
Suprabone +ASC Kit contains Suprabone –TCP granule and syringe. Suprabone-TCP is pure β-tricalcium phosphate (β-TCP). Suprabone-TCP is an osteoconductive support matrix that, when implanted, is biocompatible and resorbable, and it supports the formation of new bone tissue in the defect area. Suprabone-TCP is resorbed while being replaced by newly formed natural bone. It does not carry any diseases of human or animal source since it does not contain any tissues of that kind.
The bone marrow aspirate obtained from iliac crest or vertebral body during operation is used by mixing with Suprabone granules. Suprabone provides a perfect habitat for rehabilitative stem cells in bone marrow aspirate. The bone marrow aspirate to be obtained can be 10-20 cc, depending on the choice of the surgeon. The rehabilitation qualities of β-TCP granules, which come together with the rehabilitative stem cells in the bone marrow and rehabilitation factors, has been stated to be equivalent to autograft.
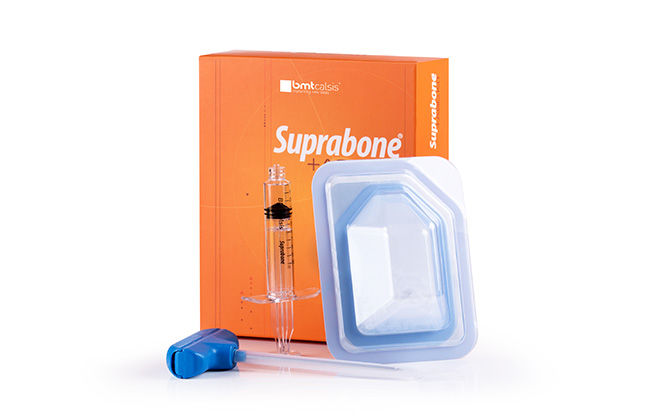
 Suprabone-TCP is pure β-tricalcium phosphate (β-TCP). Suprabone-TCP is an osteoconductive support matrix that, when implanted, is biocompatible and resorbable, and it supports the formation of new bone tissue in the defect area. Suprabone-TCP is resorbed while being replaced by newly formed natural bone. It does not carry any diseases of human or animal source since it does not contain any tissues of that kind Details…
Suprabone-TCP is pure β-tricalcium phosphate (β-TCP). Suprabone-TCP is an osteoconductive support matrix that, when implanted, is biocompatible and resorbable, and it supports the formation of new bone tissue in the defect area. Suprabone-TCP is resorbed while being replaced by newly formed natural bone. It does not carry any diseases of human or animal source since it does not contain any tissues of that kind Details…
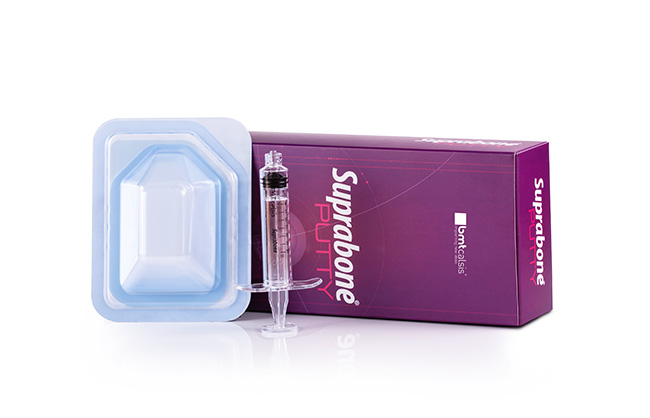
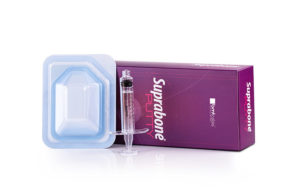 Suprabone Putty consists of two compounds provided to be merged before implementation. (Compound A and Compound B). Compound A is β-tricalcium phosphate (β-TCP), and Compound B is a cellulosic carrier. Suprabone Putty can be prepared in two different forms by mixing both of the compounds in specific proportions (refer to Implementation section below). This feature gives the surgeons the opportunity to prepare putty forms that are injectable or can be turned into dough before use, depending on the intended purpose. It is an osteoconductive support matrix that, when implanted, is biocompatible and injectable, and it supports the formation of new bone tissue in the defect area. Suprabone Putty is resorbed over time and, at the end, replaced by natural bone. The cellulosic carrier (Compound B) used in the preparation of Suprabone Putty is biocompatible and resorbed. it helps with vascularization by leaving cavities in the defect area as a result of fast degradation. Suprabone Putty does not contain tissues of human or animal sources, thus has no risk of communication of diseases. Details…
Suprabone Putty consists of two compounds provided to be merged before implementation. (Compound A and Compound B). Compound A is β-tricalcium phosphate (β-TCP), and Compound B is a cellulosic carrier. Suprabone Putty can be prepared in two different forms by mixing both of the compounds in specific proportions (refer to Implementation section below). This feature gives the surgeons the opportunity to prepare putty forms that are injectable or can be turned into dough before use, depending on the intended purpose. It is an osteoconductive support matrix that, when implanted, is biocompatible and injectable, and it supports the formation of new bone tissue in the defect area. Suprabone Putty is resorbed over time and, at the end, replaced by natural bone. The cellulosic carrier (Compound B) used in the preparation of Suprabone Putty is biocompatible and resorbed. it helps with vascularization by leaving cavities in the defect area as a result of fast degradation. Suprabone Putty does not contain tissues of human or animal sources, thus has no risk of communication of diseases. Details…

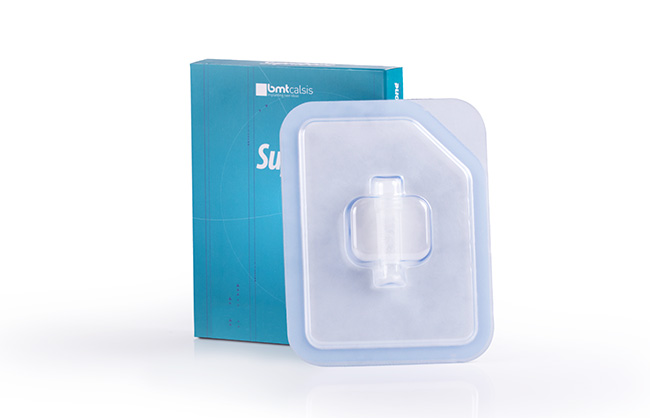
SupraFelt in a non-cellular cartilage implant in the matrix form that consists of Hyaluronic Acid (HA) and Polyglykolic Acid (PGA) for the repair and regeneration of degenerative and post traumatic cartilage defects.
SupraFelt does not contain any derivatives of tissues of human or animal sources.
SupraFelt can be implanted by being combined with products like biodegradable – biocomposite pins and tissue adhesives using arthroscopic or mini arthotomy (minimal invasive) methods in local articular cartilage defects.
Right before implantation, SupraFelt is put into, with decreasing priority, PRP (Platelet Rich Plasma), serum separated from the patient’s own blood, or sterile water for a minimum of 2 minutes so that the regeneration is sped up and the elastic structure necessary for implantation is reached. Details…
 Suprabone-TCP is pure β-tricalcium phosphate (β-TCP). Suprabone-TCP is an osteoconductive support matrix that, when implanted, is biocompatible and resorbable, and it supports the formation of new bone tissue in the defect area. Suprabone-TCP is resorbed while being replaced by newly formed natural bone. It does not carry any diseases of human or animal source since it does not contain any tissues of that kind. Details…
Suprabone-TCP is pure β-tricalcium phosphate (β-TCP). Suprabone-TCP is an osteoconductive support matrix that, when implanted, is biocompatible and resorbable, and it supports the formation of new bone tissue in the defect area. Suprabone-TCP is resorbed while being replaced by newly formed natural bone. It does not carry any diseases of human or animal source since it does not contain any tissues of that kind. Details…
Bone Graft Products
![]() This project has been financed by European Union and Republic of Turkey.
This project has been financed by European Union and Republic of Turkey.
This financing is provided from IPA funds allocated to Regional Competitiveness Operational Programme which is supported by EU and Republic of Turkey. The Contracting Authority is the Ministry of Science, Industry and Technology – DG for EU and Foreign Affairs – Regional Competitiveness Operational Programme Coordination and Implementation Directorate, and the End Recipient is the Scientific and Research Council of Turkey (TÜBITAK)